3 min read
Streamlining Bioanalytical Testing with a Unified LIMS and ELN Solution
Bioanalytical testing is a cornerstone in pharmaceutical development, providing crucial data on pharmacokinetics,...
Add text here
Add text here

Flexible and customizable LIMS combines the features in a traditional laboratory information system (LIS) with additional integrated capabilities that address the needs for clinical research and biobanking.











LabWare supports clinical laboratory automation and paperless operations with native tools that simplify the task of interfacing with a variety of laboratory analyzers and robotics platforms as well as a configurable rules engine that can be used to enable, expedite, and increase the efficiency and effectiveness of the laboratory operation.

Address all clinical trial designs from simple to complex global studies with LabWare's flexible clinical protocol definition and management tools. Configurable blinding features control data access in reports as well as the user interface.

LabWare’s Storage Location Manager provides a rich graphical interface that displays the selected storage location hierarchy. The interface provides a convenient way to visualize storage locations and to quickly identify available storage space at any level of the hierarchy tree.

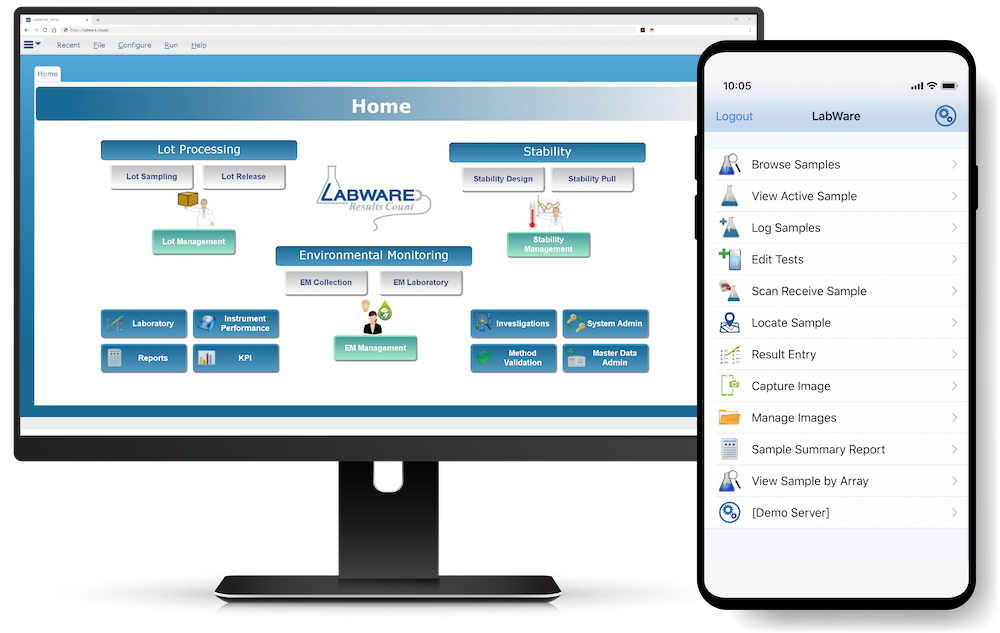
LabWare MOBILE (available on Android and iOS devices) allows users to easily connect to their LabWare systems, see their data, and perform customer-defined tasks. Learn More >
The LabWare Biorepository solution includes powerful features that enable the biobank to ensure compliance with the requirements detailed in the study protocol. Configurable templates define the study in such a way that the system can automatically drive the workflow activities, enabling validation of the data that is collected, and controlling the information and format used on any reports or in data extracts.
Additional capabilities include management of the design, production, and inventory tracking of sampling kits; kit component inventory; and requisitions and order/fulfillment management for the kits and kit supplies.
Authorized users can manage subject details including demographics, medical history, consent, and family relationships with granular control over individual data access and functional privileges. These permissions protect subject data and comply with security and privacy requirements.
Investigator sites can access the LabWare application through a web browser to pre-register specimens. You can configure the system to cater for a variety of different types of specimen or subject data that must be captured. Templates enable mapping the data in manifest files to standardized values used by the biorepository.
LabWare tracks each shipment to enable chain of custody tracking of each item. Web services interfaces are available and can be implemented to track incoming shipments by delivery service organizations such as FedEx and UPS. Each item received in the shipment can be scanned with a barcode reader and placed into a position in a storage container.
LabWare’s configurable workflow management tools provide capabilities to manage all aspects of biospecimen processing and quality control, including:
LabWare’s plate management and Electronic Laboratory Notebook (ELN) modules work together seamlessly to streamline and automate the preparation and testing of multi-well plates for a variety of workflows (ELISA, DNA extraction, sequencing, etc.).
LabWare’s powerful Rules Manager enables the lab to create and manage conditional logic for flagging, alerts, reflexes, comments, auto verification, and reporting units.
Researchers request specimens from the biorepository to support their research efforts, and the LabWare solution provides the means to track these requests using a structured request management workflow. Each request is tracked throughout its lifecycle, enabling automated interaction between the requester and the other personnel and processes involved in the biorepository’s operation.
LabWare’s biorepository solution combines powerful specimen tracking and logistics capabilities with flexible searching and specimen testing and workflow management features.
LabWare simplifies the total lifecycle management for each specimen including collection, pre-registration, receipt, testing, QC, storage management, request, retrieval, and distribution to the requestor.
Biorepository customers love:


LabWare’s clinical research solution enables pharmaceutical and biotech companies, public health labs, biorepositories, government research institutions, universities and hospitals to streamline and optimize their efforts in translational research and personalized medicine. LabWare makes key clinical trial information accessible and usable for all authorized clinical trial process stakeholders—both internal and external to your organization.
Clinical Research customers love:

LabWare’s Bioanalysis Solution avoids costly integration of a rigid bioanalytical LIMS with third-party ELN and reporting tools, often resulting in separate databases, overlapping capabilities, different vendor support channels, and ongoing integration challenges.
The fully integrated LabWare Bioanalysis Solution reduces direct software, maintenance, and infrastructure costs and the hidden costs of integrating multiple systems.
Bioanalysis customers love:



→ 4 indicators that your lab is ready for a new LIMS
→ Breakdown of LIMS deployment options (SaaS v. PaaS v. Self-hosted)
→ 8 factors that determine the total cost of ownership of your LIMS


→ 5+ technical innovations built in LabWare 8
→ Visualizations of your instruments and systems interacting with LabWare 8
→ Overview of LabWare Mobile that brings portability to lab automation

Bioanalytical testing is a cornerstone in pharmaceutical development, providing crucial data on pharmacokinetics,...
The digital revolution is transforming industries worldwide, and the petroleum sector is no exception. A landmark...
Green Valley Analyticsis a full-service independent analytical testing laboratory fully licensed by the Massachusetts...