LIMS Traceability Quality: Boosting Lab Performance
Traceability and quality are indispensable in the realm of laboratory information management systems (LIMS), with a view to sustaining precision and...
3 min read
LabWare : June 28, 2022
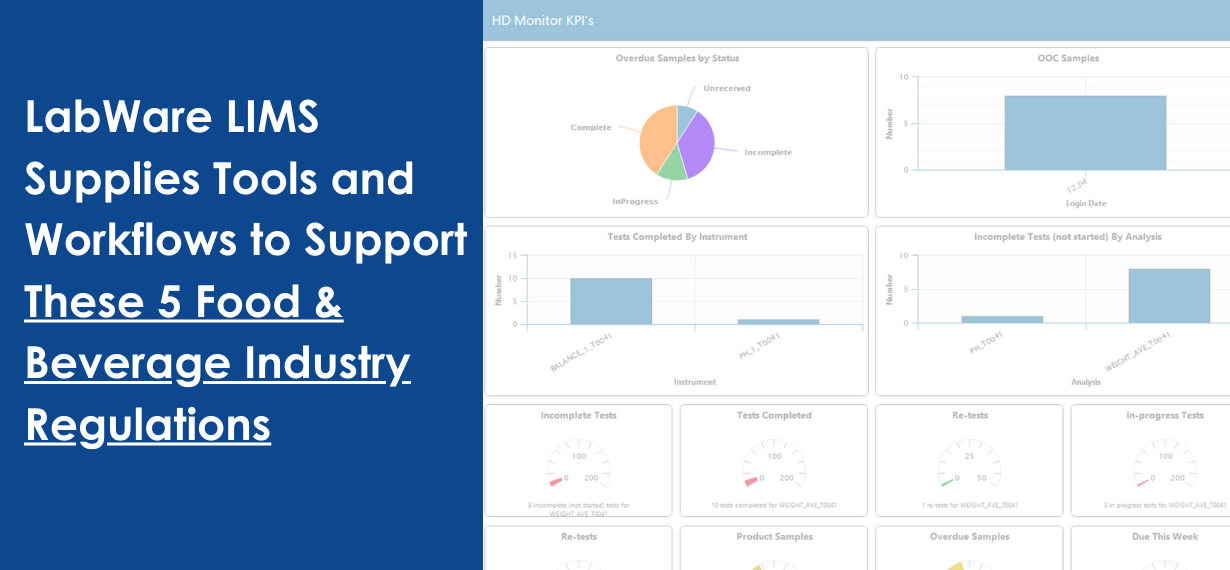
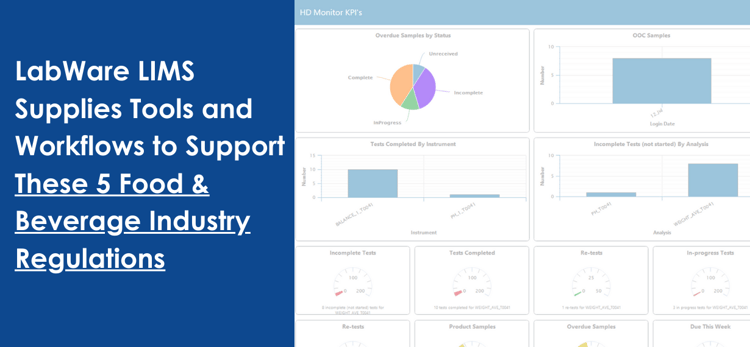
Detailed criteria regulate production environments in the food and beverage industry. These regulations are in place to ensure that consumers are safe and that quality is consistent across product batches.
Both local and international standards provide best practice information to guide food manufacturers toward regulatory compliance. Consumer demand for safe and high-quality products in a global supply chain mandates sustainable food and beverage regulation.
The International Organization for Standardization (ISO) was founded in 1947 and is present in 167 countries. Many countries have specific government organizations to protect and promote public health through the control and supervision of food safety. In many cases, these government entities may harmonize their quality system regulation with the ISO standards.Food and beverage companies that adhere to these standards require advanced data collection and reporting utilizing specific software solutions in their analytical laboratories.. Creating a compliant food and beverage production facility allows you to safely supply local and global markets with a safe and steady stream of goods. Many companies find that utilizing a laboratory information management system (LIMS) software platform helps maintain these high standards and consistency across batches.
Food service franchises and restaurants can find it challenging to comply with many regulations. Violations can be costly or dangerous; many food safety regulations carry significant penalties for noncompliance. Any restaurant that fails to maintain proper procedures can result in expensive illness or injury for staff members or guests. Owners and managers who fail to stay informed of laws that apply to their restaurants can put themselves, their staff, and their customers at risk.
Here are five food and beverage regulation considerations that are important for businesses in those industries to keep in mind:
A certification body must be competent to perform tests and calibrations, including sampling, according to ISO/IEC 17025. Tests and calibrations using standard and nonstandard methods, as well as laboratory-developed methods, are included. Accredited laboratories can demonstrate their technical proficiency and ensure that their data are accurate and precise. Third parties review the accreditation requirements to ensure that the laboratory’s quality management system continues to be technically competent and compliant with ISO/IEC 17025, even though it is voluntary.
All food producers are responsible for the safety of their products to ensure the well-being of their consumers. ISO 22000 addresses this responsibility. Unsafe food can have serious consequences. As well as working alongside other management standards, such as ISO 9001, ISO’s food safety management standards allow organizations to identify and control food safety hazards. Providing reassurance within the global food supply chain, ISO 22000 can be applied to all types of producers, helping products cross borders and bringing consumers food they can trust.
Similarly, HACCP is a management system where food safety is addressed by analyzing and controlling biological, chemical, and physical hazards at each step of the supply chain, from raw materials to manufacturing, distribution, and consumption. Management must strongly support the HACCP concept for a HACCP plan to be successful. When top management is committed to HACCP, employees feel the importance of producing safe foods.
Instituted by the U.S. Food and Drug Administration (FDA) in 1978, good laboratory practice or GLP is a formal regulation affecting the pharmaceutical and food industries. In addition to regulating nonclinical laboratory safety studies (e.g., animal toxicology testing), GLP stipulates that food and color additives, animal food additives, human and animal drugs, and medical devices are safe for use. By adhering to GLP, food laboratories can protect public health by maintaining data integrity and quality during testing.
The FDA’s Food Safety Modernization Act (FSMA) transforms food safety with a system that prevents food-borne problems by regulating the way foods are grown, harvested and processed. Facilities must evaluate the threats that could affect food safety and then specify, monitor, and maintain routine records of controls to minimize or prevent the hazards. The organization must have a corrective action plan to ensure an effective, documented resolution process when issues arise.
Part 11 of Title 21 of the Code of Federal Regulations establishes regulations for electronic records and electronic signatures (ERES) by the FDA. The guidance defines the criteria so that information submitted electronically is considered trustworthy, reliable, and equivalent to paper records.Part 11 requires organizations to adopt controls, including audits, system validations, audit trails, electronic signatures, and documentation for software and systems used to process electronic data required by FDA predicate rules. An FDA predicate rule is any requirement outlined in the Federal Food, Drug and Cosmetic Act, the Public Health Service Act, or any other FDA regulation other than Part 11.
At LabWare, we understand the importance of keeping in line with these regulations in the food and beverage industry and know that our software can help. Integrating LIMS with food laboratory instruments, such as chromatography data systems, eliminates manual labor by automating repetitive tasks. In addition, the LIMS allows food manufacturers and contract laboratories to integrate across platforms with a secure electronic data delivery system that makes up a unified database to enhance collaboration.

Traceability and quality are indispensable in the realm of laboratory information management systems (LIMS), with a view to sustaining precision and...

Today, in the rapidly changing lab setting, quality control of LIMS is essential for attaining precise and dependable analytical data. A robust...
In the world of laboratory management, LIMS audit compliance is a crucial aspect that can greatly impact an organization's success. With increasing...