Headline
Add text here
Headline
Add text here
Add text here
Add text here

Laboratories
Companies
Countries
Samples Per Year Tested Using LabWare
Daily Users
Recommend LabWare
By paying close attention to customer needs, making effective use of key technologies, and serving as a reliable and trusted partner, LabWare has emerged as the clear industry leader in laboratory automation.
In recent years, hundreds of companies and organizations all over the world have selected LabWare to be their solutions provider. In projects large and small, local and global, routine and complex, LabWare has realized success by meeting commitments and exceeding expectations.
LabWare’s Enterprise Laboratory Platform is a unique and proven suite of product capabilities that encompass LIMS (Laboratory Information Management System), ELN (Electronic Laboratory Notebook), and LES (Laboratory Execution System) in an integrated and enterprise-ready solution.
Why do many companies in a variety of industries come to the same conclusion?
One simple reason: Results count!
All enhancements for LabWare LIMS and ELN are ultimately driven by the needs of the customers, which are articulated directly through the online Wish Track feature request system or indirectly through the implementation consultants working with the customers. We are continuously developing the tools and modules to satisfy the ongoing needs of our customers. We develop them in a way that is configurable and generic so that all the customers can benefit from improvements and advancements asked for by specific customers.
We sometimes hear the criticism that LabWare LIMS is an unnecessarily big product since it tries to be everything to everybody; no laboratory will need all the available features. Features are hidden and can be exposed only if and when they are needed. LabWare also prepares industry-focused template solutions that serve as a pre-packaged starting point for project implementations.
For example, it is true that a steel plant laboratory is unlikely to perform stability studies that many pharmaceutical laboratories do. However, the effort put in by LabWare in conjunction with the pharmaceutical industry produced an excellent implementation of secure reporting that is being used by a steel company for distributing their certificates of analysis.
There are many other examples, including:
Though not every new development is going to be of interest to every laboratory, there are so many new developments that many of the developments will be of use at no additional cost.
LabWare SaaS LIMS is cloud-hosted and accessible on any browser on any device. It contains the same rich feature set as the original LabWare LIMS and comes fully validated with pre-configured best practice workflows enabling rapid deployment. LabWare SaaS LIMS is best suited for small-medium sized testing environments who want the power, compliance, and efficiency of LabWare but need the system up and running quickly and affordably. Currently LabWare SaaS is not available in all regions. Check with your local office for availability in your region.
All LabWare products are complemented by a dedicated support team, available at all times. Customers communicate with a single point of contact, regardless of whether the issue is related to the infrastructure or the solution. Proactive monitoring is used to detect and resolve problems before they occur.
LabWare products include a 99.5% availability guarantee, to ensure continuous access to the system around the clock. Our infrastructure is optimized for the LabWare products, in order to provide the user with a responsive and reliable experience.
Yes. LabWare is deployed in many large and small pharmaceutical companies which are regularly audited. LabWare is a trusted provider to companies under the most stringent regulatory scrutiny and contains functionality that meets the most recent regulation requirements and guidelines for data integrity.
LabWare QA/QC is a Software-as-a-Service LIMS offering built on 30 years of industry experience to produce a product that delivers industry best practice out of the box to companies both small and large. For customers that require more customized solutions, we have Platform-as-a-Service and Self-hosted options that we deploy at labs of all types and sizes around the world.
LabWare continues to deploy solutions that meet the challenging regulatory requirements for all the industries we support. We are constantly evolving to ensure we meet the future needs in regulatory environments that continue to become more data- and system-centric.
In association with a customer’s procedures and quality systems, LabWare offers a configurable solution that can provide a compliant and validated environment for the functions used. With comprehensive auditing, review functionality, and system controls, these implementations have been shown to meet the demands of agencies such as the FDA, MHRA, and WHO in GMP and GLP laboratories.
With the addition of our specific regulatory data integrity module (RDI), many of our customers compliance needs are now available as standardized out-of-the-box functions, with detailed and comprehensive options for controlling auditing, review, electronic signatures, data recording, data importing and more. With the RDI Guideline for the Pharmaceutical Industry, available to our customers at implementation, it is easier than ever to build an environment that customers can feel confident in.
LabWare is built to ensure all aspects of the ALCOA+ principles can be met by our customers, allowing all data to be captured and maintained appropriately.
A = Attributable - who performed an activity and when
L = Legible - can all the data (electronic and paper) be read with no ambiguity
C = Contemporaneous - documented at the time of the activity
O = Original - original observations or a certified copy
A = Accurate - no errors or modifications without appropriate documentation
“ + ”
Complete - all records exist for the respective sample(s) and batch
Consistent - the sequence of activities and events follow an expected sequence
Enduring - records are recorded on an authorized media
Available - records are accessible over the lifetime of the record (i.e. record retention period)
LabWare’s ongoing focus on these principles has resulted in a proven track record of our customers successfully meeting regulatory expectations through their use of LabWare for their LIMS and ELN needs.
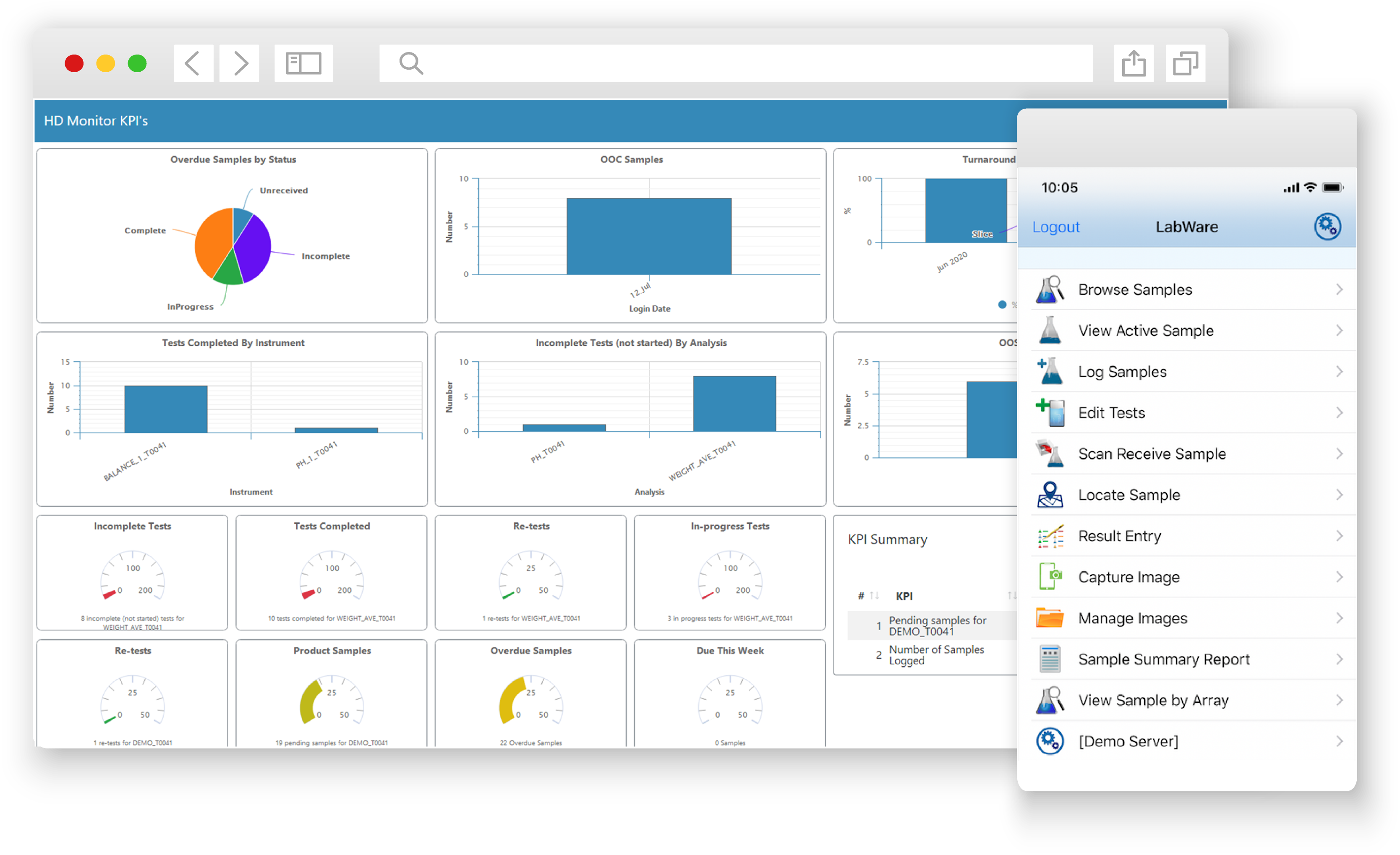
LabWare leverages state of the art technology built on a platform that enables seamless modular-based deployment of functionality to customers, leveraging our industry unique approach to ensure the longevity and upgradability of all our customers systems.
This means upgrades are driven by the customers desire to increase functionality, and not driven by changes in the technology itself, ensuring value and avoiding non-value added system upgrades.
LabWare’s unique approach to Laboratory Automation has ensured its position as the world's #1 LIMS by enabling us to be the most functional, configurable, easily integrated solution, delivering the most value to customers with the robust and future focused solution that they need.
LabWare’s flexibility means it can be used in any environment
Our architecture ensures compatibility, maintainability and upgradability.
One of the many differentiators for LabWare is our modular approach which is key to LabWare’s product design, development and release model.
LabWare LIMS and LabWare ELN are intended for use in regulated laboratories. Our QA processes are designed to ensure compliance with its specification and to be consistent with the principles outlined by GLP, GALP, cGMP, and ISO. LabWare LIMS and LabWare ELN are designed in accordance with GAMP and ISO 9001:2000 guidelines. They operate in such a way that:
Development of LabWare LIMS and LabWare ELN uses a fully documented spiral lifecycle development methodology. LabWare’s quality system consists of standard operating procedures (SOPs) that govern the entire development lifecycle and online systems that control and document all software changes. The quality system is continuously improved and audited extensively by LabWare’s customers and our internal ISO-trained auditor.
We extensively test LabWare LIMS and ELN prior to each software release and utilize formal test plans and scripts. A "traceability matrix" is used to cross-reference functional requirements to user documentation and test plans. An external validation consultant reviews executed scripts and signs off on the release to assure that the system does what it purports to do.
Every version of LabWare LIMS and LabWare ELN has been audited by numerous companies against ISO and GAMP guidelines, which reduces any validation concerns our customers have had. In addition, no LabWare customer has been unable to validate the installed system.
LabWare is open to audits by any customer or regulatory agency, and all quality system documentation is available for inspection and review.
Since its inception, LabWare's philosophy has been to put the customer first. This has been accomplished by tackling an extremely complex application and making it as simple to configure as possible. Over the years, our product has grown with the help of input from hundreds of interested parties from all parts of the globe and from every kind of laboratory.
Two principles stand out when understanding LabWare's philosophy for developing our Laboratory Information Management System (LIMS) product.
Whatever needs to be achieved must be configurable, rather than customized, by whoever needs to implement it.
Let us take a moment to understand what this philosophy means. Configuration means that standard tools and mechanisms within the product will be used to accomplish a desired effect. This involves the use of templates and may require that a scripting language be utilized—in LabWare LIMS, this language is called LIMS Basic. This scripting language generally allows the user to call standard functions within the application, and these functions will necessarily behave in a predictable and correct manner.
For example, logging a sample into LabWare LIMS using a LIMS Basic function will have exactly the same effect as logging the sample through one of the login screens. The audit trail will be produced, any configured triggers and rules will take place, and all security features configured will be used.
The alternative to configuration is customization. Typically customization involves using software tools to alter the application to suit the situation at each individual site. This approach has several potential risks. The behavior of the customized code may not be the same as that of the original code. If the person performing the customization does not completely understand the inner workings of the product, it probably will not produce the same behavior. With customization, it is easy to bypass necessary security measures, which can be particularly serious for regulated or accredited labs. Using customized code also runs the risk of preventing or at least complicating future upgrades.
All enhancements are ultimately driven by the needs of our customers, which are either articulated directly, or indirectly through the consultants working with our customers.
LabWare continuously develops the tools and modules to satisfy the ongoing needs of the customers, but do so in a way that adheres to the first principle (everything is built to be configurable and generic), so that all the customers can benefit from improvements and advancements that are initially requested by specific customers.