3 min read
Practical eQMS + LIMS: CAPA, Investigations, Training, and Document Control in One System
Most labs do not have a "quality problem." They have a systems problem: quality events live in one tool, training in...
Add text here
Add text here












LabWare LIMS organizes the tracking, testing and reporting of batches and lots to maximize laboratory efficiency. Track the status of all batches/lots tested from submission through to reporting. With built-in laboratory KPIs, data review functionality and lot release functions as well as automatic COA report generation and report approvals for each lot.

LabWare supports laboratory automation and paperless operations with native tools that simplify the task of interfacing with a variety of laboratory analyzers and robotics platforms as well as a configurable rules engine that can be used to enable, expedite, and increase the efficiency and effectiveness of the laboratory operation.

Flexible and reliable controls for managing sampling and testing. Schedules can be programmed into the system to automatically log samples and prompt regular point monitoring, ensuring all the required samples are taken and tested appropriately. Samples can also be taken manually to facilitate unscheduled sampling or personnel monitoring.

LabWare's integrated electronic laboratory notebook provides additional capability to manage large data sets in plate based testing and flexibility to document research experiments.

Highly flexible Stability Study Management capabilities coordinate and manage all related work in an entire study with one or many protocols. LabWare functionality includes inventory and storage location management, stability pulls, work assignment, sample chain of custody, results entry, review and reporting.

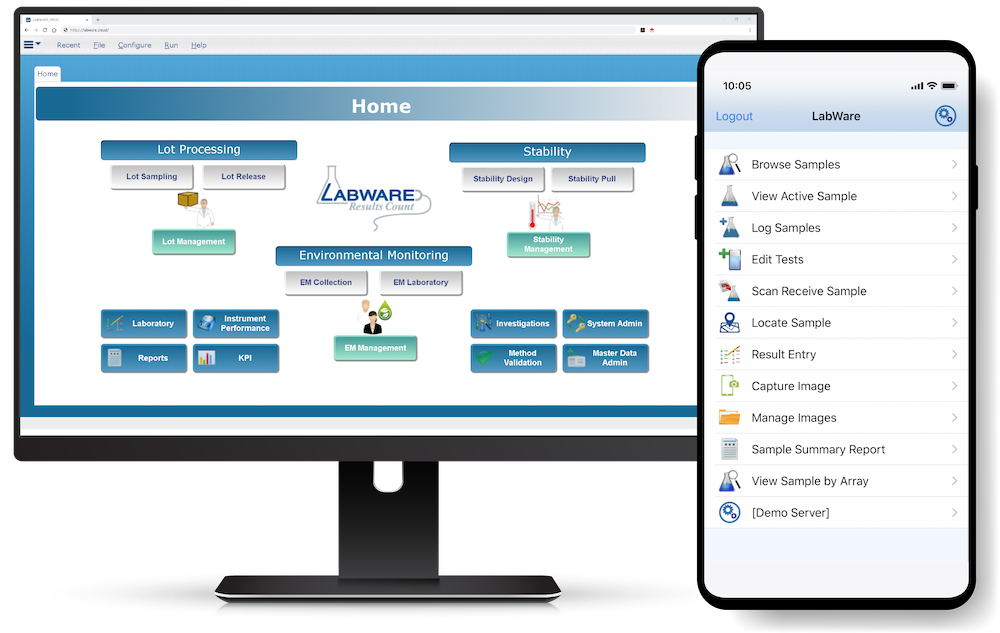
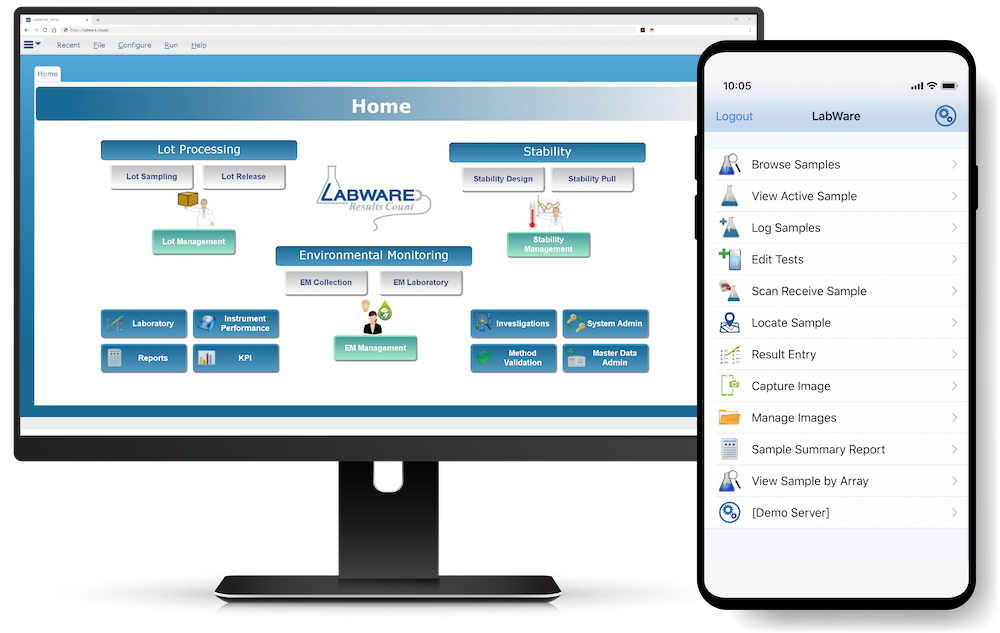
LabWare MOBILE (available on Android and iOS devices) allows users to easily connect to their LabWare systems, see their data, and perform customer-defined tasks. Learn More >
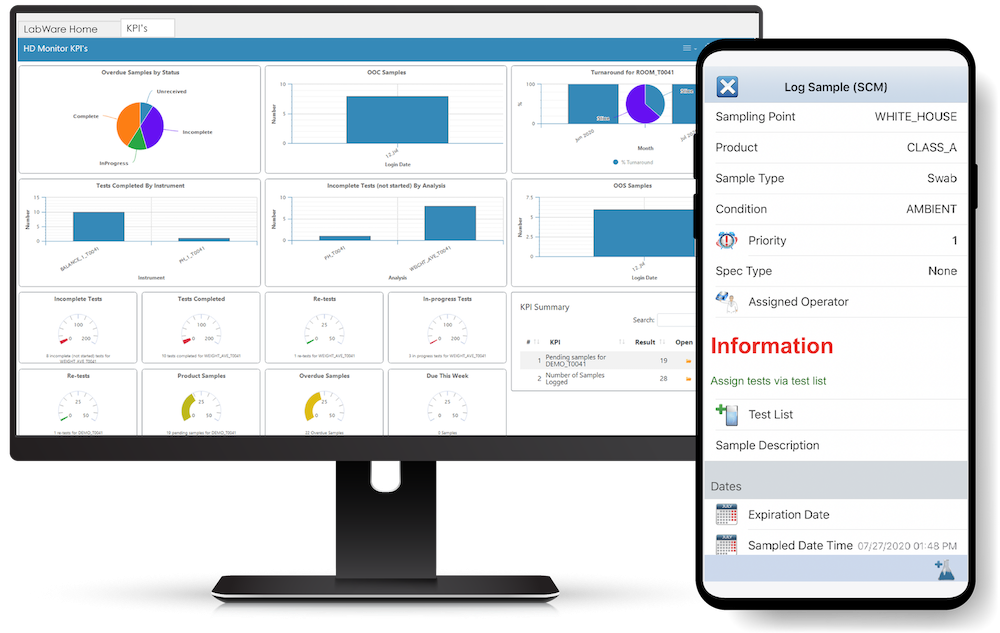
Configurable user dashboards are used to provide display of key process indicators in a variety of graphical and tabular formats. These dashboards can be personalized to fit the needs of any user role including laboratory managers, analysts, quality control personnel, etc.
Both QC and R&D laboratories in the pharmaceutical industry trust LabWare to bring products to market safely and efficiently.
LabWare’s Enterprise Laboratory Platform (ELP) is designed to be highly configurable and can be tailored to meet specific industry requirements by using a combination of purpose-built software modules along with LabWare’s pre-configured template solutions that enable rapid implementation, validation, and deployment of Life Science solutions throughout your enterprise.
Our solutions have been equally deployed across the R&D continuum offering tremendous flexibility and rapid configurability, as well as in Quality Control, ensuring sustainable regulatory compliance and quality.
The LabWare ELN (electronic laboratory notebook) product offers paperless method execution for GMP samples being tested by the QC laboratory against standard operating procedures (SOPs) and standardized testing methods (STMs). The LabWare ELN is inherently integrated into LabWare LIMS, providing seamless access to all quality data, product specifications and control limits, training certifications, instrument calibrations, and standard or solution data that ensure methods are executed by QC analysts correctly the first time.
LabWare ELN also offers a Compliance View that actively monitors and ascertains a method's compliance status as the QC analyst performs each step. Supervisors can be immediately notified when a deviation or adverse event occurs in the lab and provide immediate corrective action so that problems are detected at the source in near real-time, rather than later in the process when they are more costly to correct.
One of the most compelling attributes of the LabWare LIMS and ELN solution is its ability to be rapidly tailored through configuration, not customization, to meet your specific needs in the lab and institutional requirements like system interfaces to crucial business software and in-house databases.
LabWare provides more than 250 purpose-built software modules that enhance the LabWare core LIMS and ELN foundation and allow it to be specialized to meet unique needs. The LabWare platform offers unmatched out-of-the-box functionality in the market. LabWare’s advanced software architecture enables the product suite to be extended without requiring you to move to the next version or re-validate the system, an overwhelming advantage that most LabWare customers find genuinely compelling.
You get tremendous extensibility through configuration. Configuration allows your organization the freedom to tailor our application to meet your specific needs without worrying that it might affect your support or upgradability.
The LabWare LIMS and ELN platform are fully compliant with all technical controls of industry regulations, such as 21 CFR Part 11 (Electronic Records and Electronic Signatures), GMPs, and regulatory requirements from many international regulatory agencies. The LabWare Platform has an extensive audit trail and can be configured very easily to support your company’s interpretation of audit reason prompting and if appropriate electronic signature.
LabWare’s software is built to interoperate with virtually any type of instrument software, business software, even including custom in-house developed databases and software you have within your organization.
We offer out-of-the-box software modules that interface with many typical software applications and instrument systems used in the pharmaceutical industry, such as ERP and QMS systems such as SAP and TrackWise. You also get access to a full suite of tools that allow you to create one way or bi-directional interfaces to other systems. LabWare’s adherence to industry standards and open architecture includes web services. You will never be locked out from creating a point-to-point interface solution or connecting the LabWare platform to an Enterprise Service Bus product.
LabWare eliminates silos of information with an open architecture and powerful interoperability tools that enable exchange of information with other enterprise information systems via web services, HL7, ASTM, file transfer, and direct database communications.

LabWare’s solution for the Pharmaceutical and Biopharmaceutical industries has been developed through long term partnerships with over 2500 active customers to deliver the #1 LIMS/ELN Platform in the world, and the most functionally rich, flexible, and robust laboratory automation platform available.
LabWare provides a world-class solution for all aspects of pharmaceutical quality control (QC) and quality assurance (QA) testing. This includes comprehensive laboratory automation solutions for finished goods testing, including final certificate of analysis production (CofA), raw material testing, in-process batch testing, and the ability to perform automated in-process testing in the manufacturing rooms during production runs, if desired.
LabWare LIMS provides comprehensive out-of-the-box solutions for stability study management and environmental monitoring applicable to sterile and aseptic manufacturing facilities. Our solution can drive value from virtually every stage of the QC and QA process because the LabWare system comprehensively manages and tracks all aspects of a product’s quality.
LabWare has proven to provide dramatic reductions in product cycle times through the QC laboratory and EM/Micro laboratories, and significant reductions in adverse events such as laboratory investigations due to human error.
Business analytics, annual product review reporting, SQC and SPC trending, and key performance indicators, can be easily derived from the LabWare ELP, allowing your business to measure quantitatively QC turnaround time, investigations and out of compliance issues, and resource and instrument utilization. You can leverage this information as an asset to capacity plan and optimize your business to achieve maximum operational efficiency.
Standardizing on LabWare’s strategic platform will give your QA/QC organization the following benefits:
LabWare provides solutions for many facets of pharmaceutical R&D, including Discovery, Pre-Clinical and DMPK, Analytical Development, Chemical Development, Clinical Trials Management, and labs that support pilot plant production allowing your organization to manage a single technology platform across the R&D continuum.
The key to LabWare’s historical success as a solution in R&D comes from the tremendous breadth of product functionality rapidly configured to support virtually any scientific process.
Standardizing on LabWare’s strategic platform will give your R&D organization the following benefits:
The LabWare Pharmaceutical R&D platform (LIMS and ELN) offers many tools for the R&D scientist, such as:
LabWare’s platform embraces industry standards, and while being very secure, it provides the freedom to data-mine the relational database, summarize and export data easily to other third-party software and statistical applications, if desired. Data trending, charting, and visualization capabilities are provided natively within the LabWare platform for most common comparisons and summarizations.




→ 4 indicators that your lab is ready for a new LIMS
→ Breakdown of LIMS deployment options (SaaS v. PaaS v. Self-hosted)
→ 8 factors that determine the total cost of ownership of your LIMS


→ 5+ technical innovations built in LabWare 8
→ Visualizations of your instruments and systems interacting with LabWare 8
→ Overview of LabWare Mobile that brings portability to lab automation

Most labs do not have a "quality problem." They have a systems problem: quality events live in one tool, training in...
Quality-focused labs don't just run one application. They rely on instruments, chromatography systems, MES, ERP, QMS,...
Anatomic Pathology labs face continuing challenges with staffing shortages, pressures to integrate complex technologies